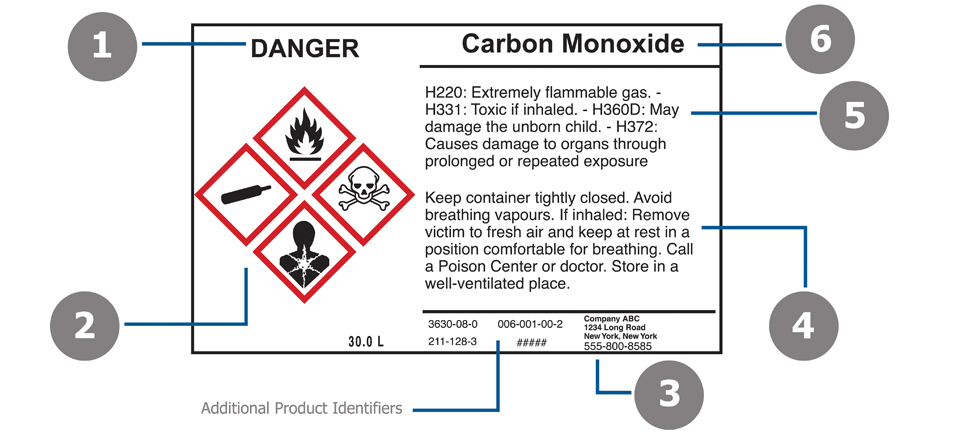
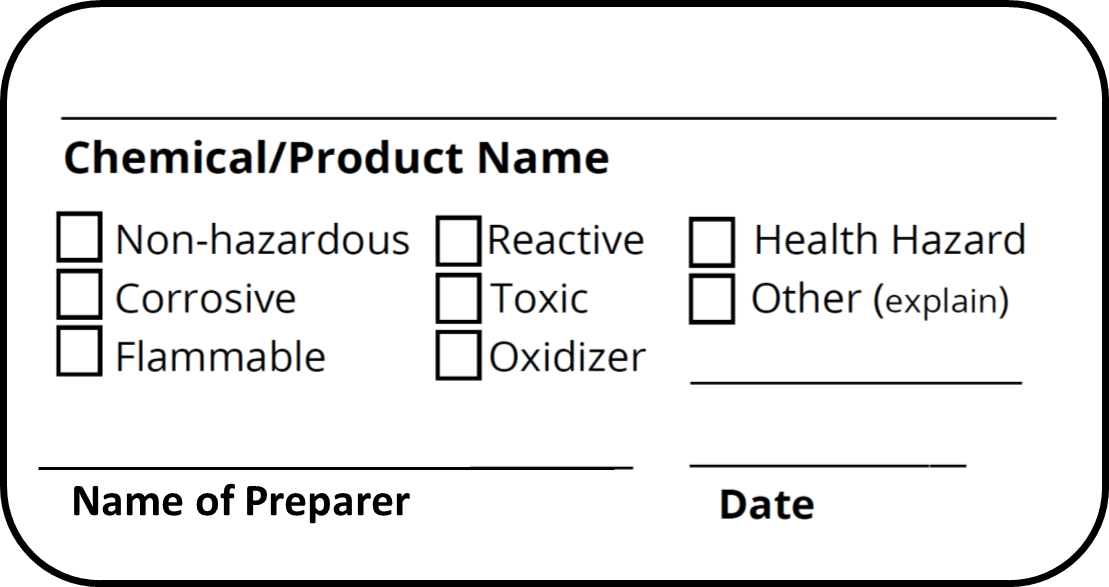
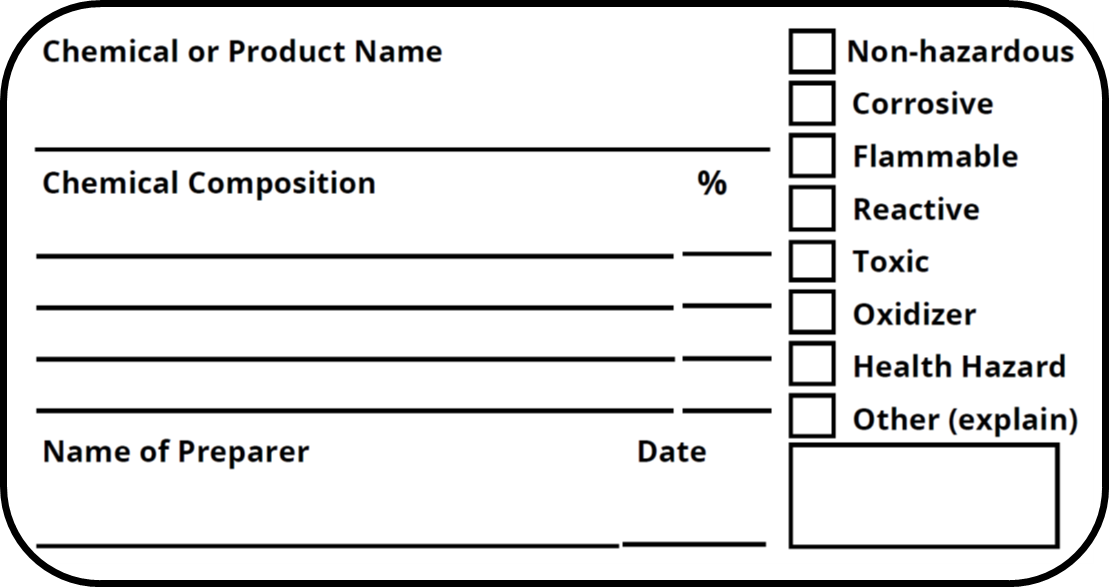
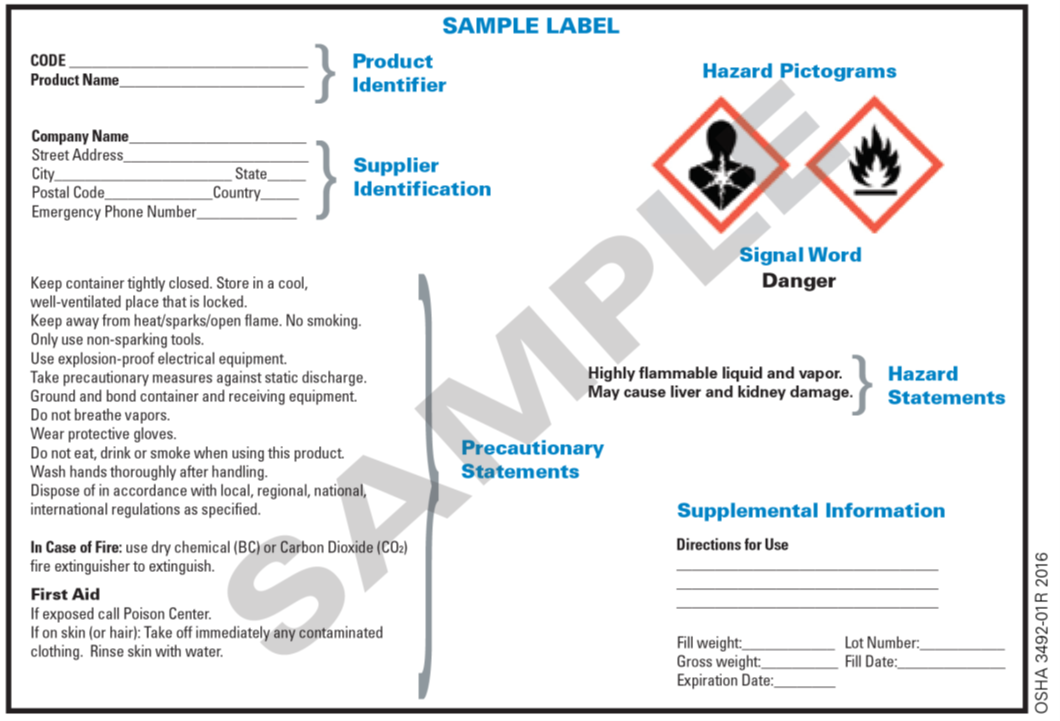
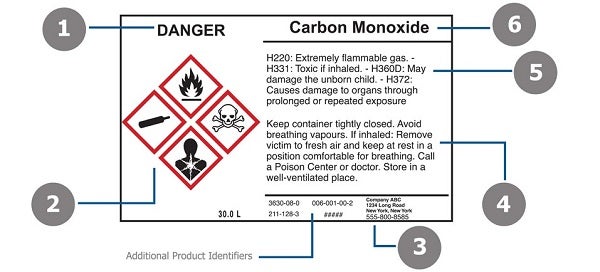
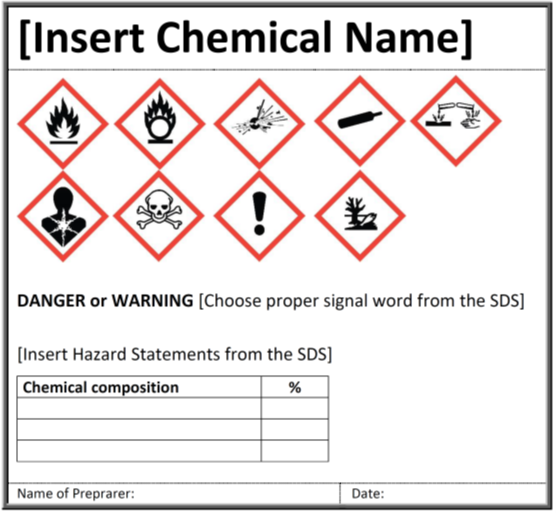
42 regulations require that product labels on containers
› laws-regs › regulations1910.1200 - Hazard Communication. | Occupational Safety and ... Any food, food additive, color additive, drug, cosmetic, or medical or veterinary device or product, including materials intended for use as ingredients in such products (e.g., flavors and fragrances), as such terms are defined in the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.) or the Virus-Serum-Toxin Act of 1913 (21 U.S.C. 151 et seq.), and regulations issued under those ... › scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (e) A food shall be exempt while held for sale from the requirements of section 403(k) of the act (requiring label statement of any artificial flavoring, artificial coloring, or chemical preservatives) if said food, having been received in bulk containers at a retail establishment, is displayed to the purchaser with either (1) the labeling of ...
› laws-regs › regulations1910.1030 - Bloodborne pathogens. | Occupational Safety and ... Red bags or red containers may be substituted for labels. 1910.1030(g)(1)(i)(F) Containers of blood, blood components, or blood products that are labeled as to their contents and have been released for transfusion or other clinical use are exempted from the labeling requirements of paragraph (g).
Regulations require that product labels on containers
› food › food-labeling-nutritionChanges to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ... › media › blogOrganic 101: What the USDA Organic Label Means | USDA Mar 13, 2019 · This is the third installment of the Organic 101 series that explores different aspects of the USDA organic regulations. Amidst nutrition facts, ingredients lists, and dietary claims on food packages, “organic” might appear as one more piece of information to decipher when shopping for foods. So understanding what “organic” really means can help shoppers make informed choices during ... › cosmetics › cosmetics-labelingShelf Life and Expiration Dating of Cosmetics | FDA There are no U.S. laws or regulations that require cosmetics to have specific shelf lives or have expiration dates on their labels. However, manufacturers are responsible for making sure their ...
Regulations require that product labels on containers. › cosmetics › cosmetics-labelingShelf Life and Expiration Dating of Cosmetics | FDA There are no U.S. laws or regulations that require cosmetics to have specific shelf lives or have expiration dates on their labels. However, manufacturers are responsible for making sure their ... › media › blogOrganic 101: What the USDA Organic Label Means | USDA Mar 13, 2019 · This is the third installment of the Organic 101 series that explores different aspects of the USDA organic regulations. Amidst nutrition facts, ingredients lists, and dietary claims on food packages, “organic” might appear as one more piece of information to decipher when shopping for foods. So understanding what “organic” really means can help shoppers make informed choices during ... › food › food-labeling-nutritionChanges to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...






























Post a Comment for "42 regulations require that product labels on containers"